Thermodynamics
Table of Contents
- Notation
- Equations / Laws
- Zeroth Law
- First Law of Thermodynamics
- Maxwell-Boltzmann distribution
- Compressibility
- Bulk modulus
- Heat capacity
- Thermal expansivity
- Bernoulli Equation
- Ideal gas law
- Work (reversible process)
- TODO Boltzmann Distribution
- Adiabatic expansion
- Clapeyron equation
- Van der Waals equation
- Lennard-Jones potential
- Ionic bonding
- Surface energy
- Capillary rise
- Viscosity
- Reynolds number
- Braggs Law
- Miller indices
- Entropy
- Internal energy
- Gibbs function
- Enthalpy
- Helmholtz function
- Second Law of Thermodynamics
- Maxwell's relations
- Definitions
Notation
 is heat / thermal energy
is heat / thermal energy is internal energy
is internal energy
Equations / Laws
Zeroth Law
If each of two systems is in thermal equilibrium with a third system they are in thermal equilibrium with each other.
First Law of Thermodynamics
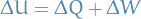
Maxwell-Boltzmann distribution

Compressibility
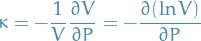
Bulk modulus
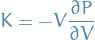
Heat capacity
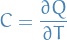
Thermal expansivity
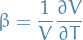
Bernoulli Equation
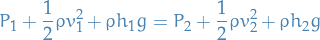
Ideal gas law

Work (reversible process)
"Derivation"

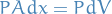
which gives us
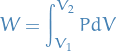
Differential

where work is defined as the work done ON the system by its surroundings .
TODO Boltzmann Distribution

In the case where we have no degeneracy (or you simply care about the probability of specific state corresponding to some energy $ε$i instead of the probability of the energy  itself, i.e. don't want to included all possible states which can take on the this energy):
itself, i.e. don't want to included all possible states which can take on the this energy):
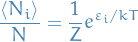
Notation
 is the expected number of particles in the energy level indexed by
is the expected number of particles in the energy level indexed by 
 total number of particles
total number of particles the energy of the ith energy level
the energy of the ith energy level is the degeneracy of the ith energy level with energy
is the degeneracy of the ith energy level with energy  (this is always an integer), which corresponds to the number of quantum mechanical observables which can take on an energy of
(this is always an integer), which corresponds to the number of quantum mechanical observables which can take on an energy of 
Derivation
In our deduction we're going to consider a microcanonical ensemble.
To begin with, we ignore the problem of degeneracy; i.e. we assume there is only one way to put  particles into the energy level
particles into the energy level  .
.
The number of possible ways to "bin" the particles in the different energies:
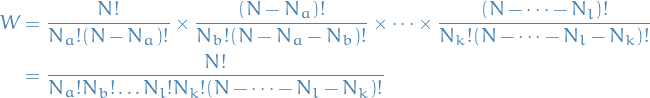
where each of the factor on the first line represents the number of possible ways to partition all  particles into groups of
particles into groups of  , then the rest of the particles partitioned into bins of size
, then the rest of the particles partitioned into bins of size  , and so on. I.e. we end up counting the number of possible ways to partition all
, and so on. I.e. we end up counting the number of possible ways to partition all  particles into the different "energy-bins" with
particles into the different "energy-bins" with  particles in the ith "energy-bin".
particles in the ith "energy-bin".
Because we're "exhausting" the number of particles, i.e. we keep "binning" until we have no particles left (each particle MUST be assigned a bin), then  , and we can write
, and we can write
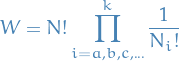
Which is just the multinomial coefficient , the number of ways to arrange  objects into
objects into  bins, ignoring order / permutations.
bins, ignoring order / permutations.
Now we want to take into account the possible degeneracy degree  of each energy level. The
of each energy level. The  corresponding to the energy
corresponding to the energy  can then be arranged in
can then be arranged in  ways within the energy-level (since for each particle with energy
ways within the energy-level (since for each particle with energy  can go in either of the
can go in either of the  "sub-bins"). Thus,
"sub-bins"). Thus,
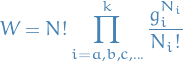
BUT when doing this we're treating the particles as distinguishable , i.e. the order of arranging into the "sub-bins" matter, which leads to a "invalid" entropy (not extensive , which means that the entropy is not proportional to the amount of substance, which it really ought to be). This is called the Gibbs paradox. This leads to the Bose-Einstein expression for 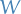 :
:
NOT FINISHED YET DUE TO REALIZING THIS NOT FITTING AS WELL INTO AN ANKI CARD AS I HAD HOPED.
Adiabatic expansion

Clapeyron equation

Which is basically saying that when we're moving between two phases, the change in pressure 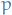 wrt. temperature
wrt. temperature  is equal to the ratio between the change in entropy and volume.
is equal to the ratio between the change in entropy and volume.
TODO Derivation
Van der Waals equation

- Weak force
- Isotropic
- Noble gases and neutral molecules
- Potential given by Lennard-Jones potential
TODO Derivation
Lennard-Jones potential
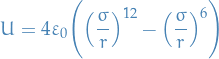
where the first term is the repulsion and the second term is the Van der Waals forces.
Ionic bonding
![\begin{equation*}
U(r) = 1.481 \varepsilon \Big[ \pm \Big(\frac{\sigma}{r} \Big) + \Big(\frac{\sigma}{r} \Big)^9 \Big]
\end{equation*}](../../assets/latex/thermo_068f28119cd13ce1ca67a45ec298f9fb77063c84.png)
- assumes single and complete ionisation, which is why we can consider the pairwise potential as the Coloumb force (1st term) together with a repulsive force (2nd term)
- attraction via electron exchanges to produces filled orbitals results in charged particles that attract
Surface energy
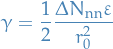
where  is the seperation energy and
is the seperation energy and 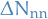 is the change in nearest neighbours .
is the change in nearest neighbours .
TODO Derivation
Capillary rise
Viscosity
Viscosity characterizes the sheer forces that exist in a moving fluid.
For a gas we have
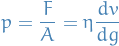
Reynolds number

Braggs Law

Miller indices
Index system tused to identify equally-spaced parallel planes intersecting lattice points.
It works as follows:
If the plane intercepts at:
 along the x-axis
along the x-axis along the y-axis
along the y-axis along the z-axis
along the z-axis
the the lattice has Miller indices 
Entropy

Or with a fixed energy, for a system in a particular macrostate , defined by the number of associated micro-states  :
:

Internal energy

This is true regardless of whether or not the process is reversible!
Gibbs function
The function
Is a state-function.

and

Criterion for spontanous process

and in equilibrium

Enthalpy
Is a state-function.
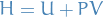
and

Helmholtz function

and

Second Law of Thermodynamics

for any process, in total. That is,
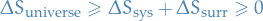
Maxwell's relations
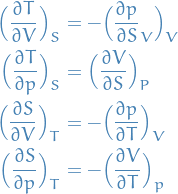
Definitions
Temperature
The temperature of a system is a property that determines whether or not that system would be in thermal equilibrium with other systems.
Equilibrium state
An equilibrium state is one in which all the bulk physical properties do not change with time and are uniform throughout the system
Young's Modulus
Young's modulus is a measure of the ability of a material to withstand changes in length when under lengthwise tension or compression.
Systems
Closed system
Cannot exchange matter with its surroundings, but may exchange energy.
Isolated system
No exchange of any material or energy with surroundings.
Reversible process
A process where every step for the system and its surroundings can be reversed. A reversible process involves a series of equilibrium states.
Path-independent integral over  wrt.
wrt.  for an ideal gas (this is true for any state variables )
for an ideal gas (this is true for any state variables )
Ensembles
Microcanonical ensemble
A microcanonical ensemble is a mechanical ensemble which represents the possible states of the particles in a system where the total energy of the system is exactly known .
Grand canonical ensemble
A grand canonical ensemble is the statistical ensemble that is used to represent the possible states of a mechanical system of particles that is being maintained in thermodynamic equilibrium (thermal and chemical) with a reservoir.
Canonical ensemble
A canonical ensemble is the statistical ensemble that is used to represent the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchage energy with the heat bath, so that the states of the system will differ in total energy.
Adiabatic process
A adiabatic process is a reversible and adiathermal (thermally isolated) process.
Bonding
Covalent bonding
Strong and directional based bonding based on electron-sharing .
Metallic bonding
Attraction of like species via sharing of free electrons.
It's similar to covalent , but the electrons are completely delocalized and free to move.
Hydrogen bonding
Hydrogen atom itself is shared between atoms.
Isotropic
A process is called isotropic if 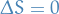 .
.