Fuel Cell
Table of Contents
Overview
Hydrogen fuel cells are units which produce electricity through a reaction of positively charged hydrogen ions with oxygen or another oxidixing agent, i.e. separating electrons from the oxidizing agent by having it react with the positively charged hydrogen ion. This is performed through the usage of a anode, a cathode, and a catalyst.
Awesome illustration of Proton Exchange Membrane (PEM) fuell cell
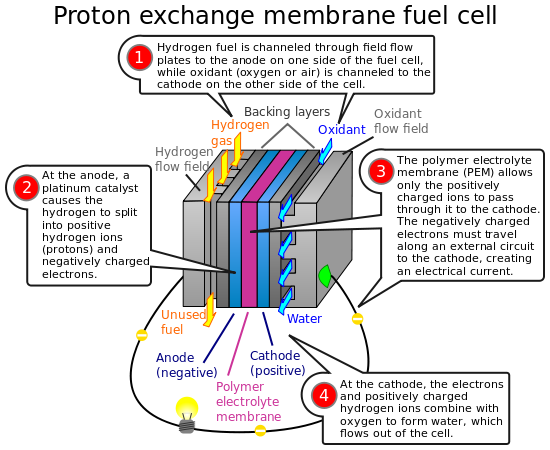
Appendix A: Words
- Oxidation state / number
- degree of oxidation (loss of electrons) of an atom in a chemical compound.
- Oxidation
- loss of electrons or an increase in oxidation state by a molucule, atom, or ion.
- Reduction
- gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.